Overview of Skeletal Muscle Tissue
Skeletal muscle tissue is the type most commonly associated with the body’s movement. Under the microscope, skeletal muscle reveals a distinct structure. This complexity is due to the specialized cells that make up the tissue. These cells are long, cylindrical fibers packed in parallel bundles.
Each skeletal muscle fiber shows unique features. You will notice a pattern of light and dark bands. This striation is characteristic of skeletal muscle under the microscope. The dark bands are known as ‘A bands,’ while the lighter ones are ‘I bands.’
Muscle fibers contain many nuclei. These are found just under the cell membrane, or sarcolemma. The position of nuclei is important in understanding muscle function and repair.
Skeletal muscles have an abundant supply of blood vessels and nerves. This supports their high demand for oxygen and nutrients. It also ensures precise control of movement.
When viewing skeletal muscle under microscope, the tissue’s complexity becomes clear. Each fiber is a powerhouse of potential energy, awaiting neural signals to spring into action.
In sum, skeletal muscle tissue is a highly organized and dynamic structure. Its microscopic anatomy is not just fascinating scientifically, but key to our understanding of movement and health.
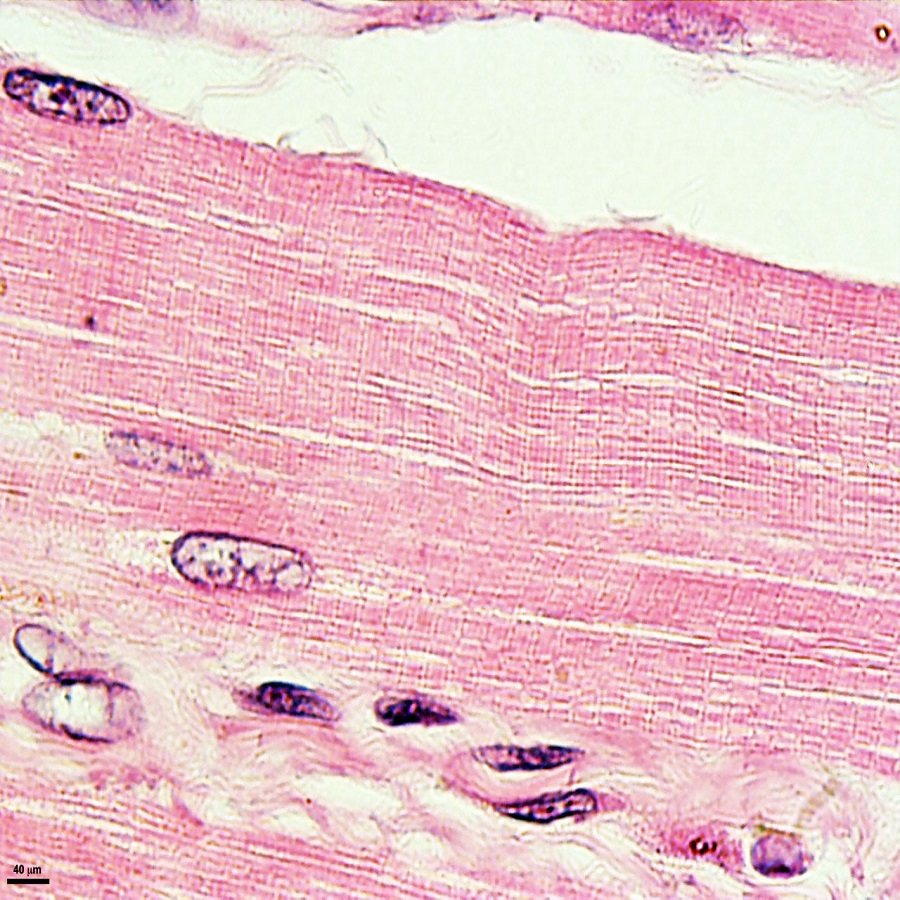
Key Characteristics of Skeletal Muscle Fibers
When examining skeletal muscle under a microscope, certain key characteristics stand out. {
Striated Pattern: Each skeletal muscle fiber displays a unique striated pattern. This is due to the arrangement of myofilaments in repeating units, creating visible stripes.
Multinucleated Cells: Unlike other types of cells, skeletal muscle fibers boast multiple nuclei. This is attributable to their origin as many single cells that fused together.
Abundant Mitochondria: The presence of numerous mitochondria in muscle fibers reflects their high energy demands. This aids rapid energy production during contraction.
Defined Sarcolemma: The sarcolemma, or cell membrane, encases muscle fibers tightly. This is vital for receiving and conducting electrical impulses.
T-tubule System: Muscle fibers exhibit a T-tubule system, essential for propagating electrical signals deep into the fibers.
Sarcoplasmic Reticulum: This specialized endoplasmic reticulum regulates calcium ion storage and release, crucial for muscle contraction.
These features play pivotal roles in the muscle’s ability to contract and generate movement, demonstrating the elegance and complexity of skeletal muscle microscopic anatomy. Recognizing these characteristics is crucial for understanding overall muscle function.
The Microscopic Anatomy of Skeletal Muscle
A deeper look into skeletal muscle under a microscope unveils its complex arrangement. This muscle type, essential for voluntary movements, displays an intricate architecture on the microscopic level.
Muscle fibers, the basic units, are organized into larger structures called fascicles. Surrounding each fascicle is a layer of connective tissue, the perimysium, which protects and supports the muscle fibers within. Collectively, fascicles form the bulk of the muscle, each wrapped by another protective layer, the epimysium.
Each individual muscle fiber is a tube-like cell filled with myofibrils. These are the powerhouses of the muscle, the structures that actually contract. Myofibrils are composed of even smaller units, the sarcomeres, aligned end-to-end. Sarcomeres are the true functional units of muscle contraction, and their repeating pattern contributes to the striated look of skeletal muscle under the microscope.
Skeletal muscles are rich in capillaries, weaving between fibers. This network ensures a steady supply of oxygen and nutrients, necessary for muscle function and endurance. Nervous tissue also intertwines with muscle fibers, delivering the signals that control contraction and relaxation.
This microscopic anatomy—not just of individual fibers but also of the network of tissues supporting them—is what makes skeletal muscle so robust and responsive. Understanding this complex structure is essential for appreciating how muscles operate and the ways they empower movement and support the skeleton.
The Role of Myofibrils and Sarcomeres
When we dive into the subject of skeletal muscle under microscope, we place the spotlight on myofibrils and sarcomeres. Myofibrils are long, filamentous structures that fill the muscle fibers. They are vital for the muscle’s ability to contract. Composed of smaller units called sarcomeres, they work as the engines of muscle movement.
Myofibrils: These thread-like components are power-packed with actin and myosin. These are the proteins responsible for muscle contraction. Myofibrils run the entire length of the muscle fiber. They ensure that contraction forces distribute evenly.
Sarcomeres: Often termed the ‘functional units of muscle’, sarcomeres form a chain within myofibrils. They repeat from one end to the other. Each sarcomere houses a precise arrangement of actin and myosin. This overlap creates the muscle’s striated appearance under the microscope.
The action starts when a sarcomere shortens. This happens as thin actin filaments slide over thick myosin ones. It is this sliding action that results in muscle tissue contracting. Sarcomeres also lengthen when muscles relax, indicating their reversible nature.
This highly coordinated process within myofibrils and sarcomeres is key to muscle function. It allows skeletal muscles to execute precise and controlled movements. It’s a process so crucial, yet only visible when observing skeletal muscle under a microscope. By understanding myofibrils and sarcomeres, we grasp the essence of muscle mechanics.
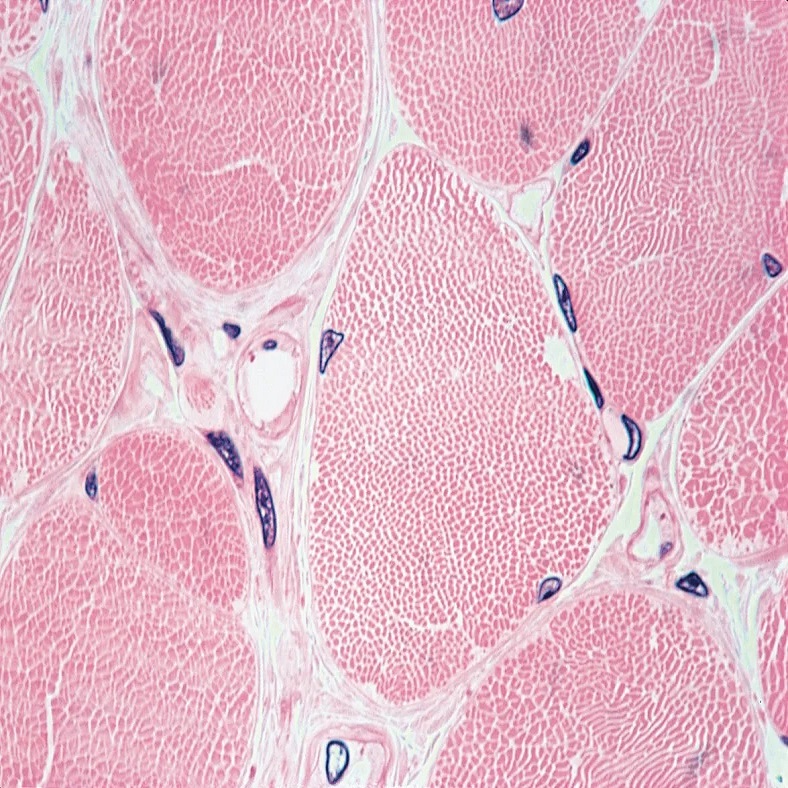
Staining Techniques for Muscle Tissue Observation
Studying skeletal muscle under a microscope requires clear visualization of the intricate structures. Staining techniques are vital for enhancing the contrast between different tissue components. This makes it possible to distinguish between the various microscopic features of muscle fibers. There are several stains that researchers and scientists commonly use for muscle observations.
Hematoxylin and Eosin (H&E): This is a widely used staining combination. Hematoxylin stains cell nuclei blue, while eosin colors the cytoplasm and muscle fibers pink. This contrast allows for easy identification of the cells’ structural details.
Trichrome Stains: These stains, such as Masson’s trichrome, color connective tissue blue or green. Muscle fibers stand out in red or pink, providing a clear differentiation between muscle and connective tissues.
Immunofluorescence Staining: By using antibodies that attach to specific muscle proteins, this staining lights up targeted areas. When viewed under a microscope with a special light, these areas will fluoresce, showcasing particular parts of the muscle fiber.
Enzyme Histochemistry: This technique reveals the distribution of certain enzymes within the muscle. It is especially useful for showing metabolic differences across the muscle fibers.
Using these staining methods enhances our understanding of skeletal muscle under the microscope. These techniques reveal intricate details that are not seen in unstained samples. Thus, they are crucial for research in muscle physiology and pathology.
Understanding Muscle Contraction at the Microscopic Level
At the heart of muscle movement lies the process of contraction, a complex dance not fully appreciated until observed under a microscope. To understand this mechanism, it’s crucial to look at two particular proteins: actin and myosin. Found within the myofibrils, these proteins interact to create the force needed for muscle contraction.
Actin is a thin filament that forms a helix, wrapping around to provide a track. Myosin, the thicker filament, has heads that bind to actin’s helix. During contraction, these myosin heads pivot, pulling the actin filaments closer together. As this occurs across myofibrils’ countless sarcomeres, the muscle fiber as a whole contracts.
Calcium’s Role: When a nerve impulse reaches a muscle cell, it triggers the release of calcium ions. These ions bind to a protein called troponin, which causes another protein, tropomyosin, to reveal the binding sites on actin. Myosin heads latch onto these sites and pull, shortening the muscle fiber.
ATP as Fuel: Adenosine triphosphate (ATP) is vital for muscle contraction. It provides the energy that allows myosin to detach and reattach to actin during the contraction cycle. Without ATP, muscles would remain in a contracted state, known as rigor.
In summary, muscle contraction is a precise sequence of events. It starts with a neural signal and ends with the sliding of actin and myosin filaments. This can only be observed when looking at a skeletal muscle under microscope. Knowing these details helps researchers understand how muscles work and what may go wrong in muscle diseases.
Common Observations in Diseased or Damaged Muscle Tissue
Skeletal muscle under microscope offers deep insights not only into healthy tissue but also into diseased or damaged muscle. When muscle tissue undergoes damage or is affected by disease, several distinct microscopic changes can be observed. Here are some common findings:
Fiber Atrophy: Affected muscles often show reduced fiber size, indicating a loss of muscle mass known as atrophy.
Fiber Necrosis: Dead muscle fibers, or necrosis, can be a clear indication of severe damage or advanced disease.
Inflammation: Inflammatory cells often infiltrate the spaces between muscle fibers in conditions like myositis.
Fibrosis: Scar tissue may replace normal muscle tissue, leading to stiffness and decreased function.
Variation in Fiber Size: Damaged muscles may display an uneven mix of fiber sizes, a sign of regeneration or ongoing damage.
Internal Nuclei: While normally found at the periphery, nuclei within fibers hint at regeneration or response to injury.
Observational studies of skeletal muscle under microscope allow us to recognize these variations. It’s vital to monitor these changes to understand the progression of muscle-related diseases.
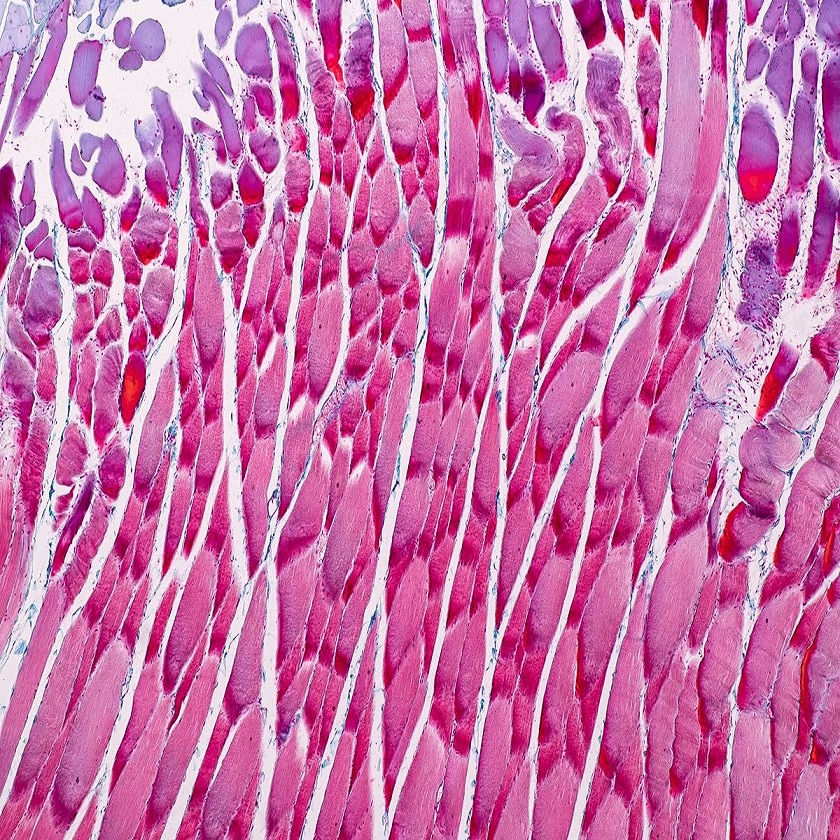
Advanced Imaging Techniques in Muscle Microscopy
With advancements in technology, various imaging methods have furthered our understanding of skeletal muscle under microscope. These allow deeper exploration into the muscular structure and function. Here’s a look at some advanced techniques being used.
Confocal Microscopy: This method provides a clearer, more detailed three-dimensional image of muscle fibers. It uses laser light to scan the sample in successive layers, resulting in high-resolution images that can reveal the organization of myofilaments within myofibrils.
Transmission Electron Microscopy (TEM): TEM offers an incredibly high magnification, exposing the ultrastructural components of muscle cells. Researchers can see detailed aspects of the sarcomeres, T-tubules, and the sarcoplasmic reticulum.
Scanning Electron Microscopy (SEM): SEM gives a surface view of muscle tissue, which is valuable for observing the overall architecture of muscle fibers and the connection between fibers and the surrounding connective tissues.
Fluorescence Microscopy: This technique uses fluorescent dyes that bind to specific muscle proteins. It enables the visualization of particular components within the muscle fiber, such as actin and myosin.
Photon Microscopy: Photon microscopy can generate images of muscle tissue without the need for dyes or stains. It can show changes in muscle tissue composition and detect early signs of muscle disease.
Multiphoton Microscopy: This advanced form of fluorescence microscopy allows for deep-tissue imaging. It can produce detailed images of muscle fibers at work without damaging the tissue.
These sophisticated imaging methods provide powerful tools for scientists and researchers. They delve into skeletal muscle’s microscopic world with precision and detail. By using these technologies, we can achieve greater insight into muscle conditions and develop targeted treatments for muscular diseases.